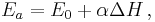Bell–Evans–Polanyi principle
In physical chemistry, the Bell–Evans–Polanyi principle observes that in a series of similar homolytic atom transfer reactions of the general type, there is often a closely linear relationship between the activation energy and the reaction enthalpy. This can be expressed as:
where:
- E0 is the activation energy of the reference reaction
- ΔH is the enthalpy of each reaction in the series
In terms of reaction energy diagrams, this indicates that for reactions having similar energy profiles, the height of the barrier will decrease as the reaction become more exothermic.
References
- Advanced Organic chemistry (part A: Structure and Mechanisms) FRANCIS A. CAREY